This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
News
Seeing the retina in a new light
A newly established CERA research unit is using groundbreaking experimental methods to uncover previously unknown structures in the retina, with hopes of improving treatments for glaucoma.
The Visual Neurovascular Research Unit, led by Principal Investigator Dr Luis Alarcon-Martinez, is investigating the mechanisms which control blood flow to the retina, a complex layer of cells at the back of the eye.
When our vision works correctly, light is picked up in the retina and turned into electrical signals which are transmitted to the brain by millions of retinal ganglion cells.
These cells transmit visual information to the brain via long nerve fibres, known as axons, which bundle together to make up the optic nerve.
To function properly, retinal ganglion cells require an adequate supply of oxygen and nutrients from surrounding blood vessels.
Inadequate blood supply can damage these cells and lead to vision loss.
Dr Alarcon-Martinez’s research aims to gain a better understanding of how exactly blood is distributed in the retina in order to prevent damage and preserve sight.
“We know that interruption of blood supply is found in diseases such as glaucoma, diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration,” says Principal Investigator Dr Luis Alarcon-Martinez, who heads the Visual Neurovascular Research Unit.
“But we currently have only limited understanding of the exact mechanisms that control the distribution of blood in the retina.”
“Our team will be investigating the processes that enable neurons to communicate with blood vessels.”
“The neurons are saying ‘I need more energy’, and the vessels supply the elements to produce it. But we don’t know exactly how that happens.”
A new experimental set-up
This lack of understanding might come as a surprise, but Dr Alarcon-Martinez says there are two good reasons for it.
The first is that the processes are happening at a very small scale: that of single nerve cells and capillaries.
The second is that they need to be observed in living organisms.
“What we are trying to understand here are dynamic processes’’ says Dr Alarcon-Martinez.
“You need to see the blood running into the vessels, and the nerve cells being activated.”
“These complications have meant that until recently, we simply didn’t have the technology to be able to look at such small environments in the retinas of living organisms.”
Dr Alarcon-Martinez was part of a team at the University of Montreal which developed a new experimental set-up to do just that.
Using cutting-edge two-photon microscopy, Dr Alarcon-Martinez and colleagues were the first to observe these processes in living organisms at such high levels of resolution.
Discovering nanotubes
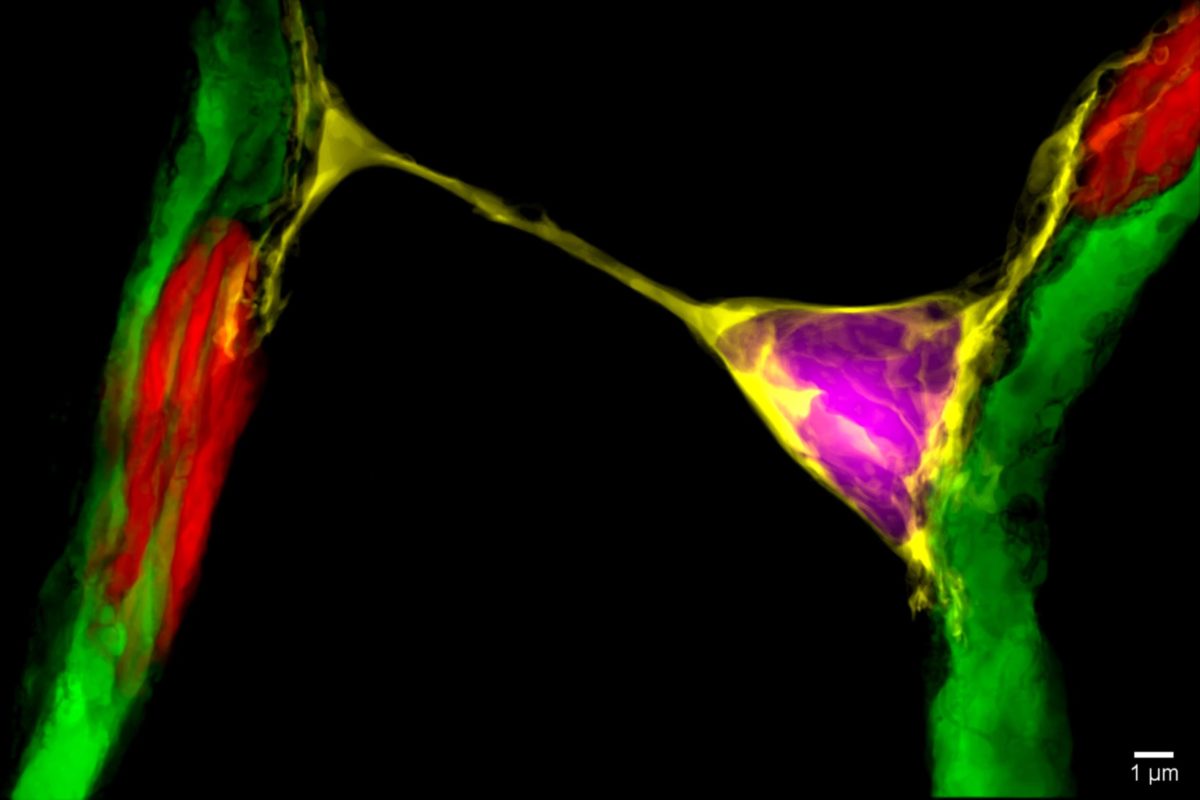
They discovered a previously unknown mechanism by which cells in the retina communicate with one another to regulate blood supply.
Embedded within the capillaries are pericytes, cells that can control the amount of blood passing through the capillaries by dilating or contracting them.
Looking at vascular changes in the retinas of mice, Alarcon-Martinez and colleagues showed that pericytes project very thin tubes – known as nanotubes – to communicate with one another and supply blood where it is most needed.
Their study, published in Nature in 2020, also showed that capillaries lose their ability to regulate blood supply when these nanotubes are damaged, with detrimental effects on neurons and the functioning of the retina.
These findings suggest that the interruption of blood supply found in retinal neurodegenerative diseases might be a result of these nanotubes breaking down.
Where to next
Future research for the Visual Neurovascular Research Unit will focus on gaining a better understanding of the dynamics between these nanotubes, the neurons, and vessels.
“We know that when a neuron is activated, things change. We want to know exactly what the neuron is releasing to make these nanotubes work.”
The hope is to help develop strategies to prevent or treat retinal neurodegenerative diseases.
Potential applications of the research are not limited to the visual system, but also include neurodegenerative diseases such as Alzheimer’s disease and strokes.
Dr Alarcon-Martinez says he’s excited to join the CERA research team.
“I’m delighted of course to be joining one of the very top ophthalmology research centres in the world,’’ he says.
“But just as important for me is the inclusion of clinical research with basic science. We do the work we do to help people, and I can’t think of a greater reward than seeing the impact our research can have on patients.”
More info
Read the full research article: Alarcon-Martinez, L., et al. (2020). Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature, 10.1038/s41586-020-2589-x.

