This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Research
Implant’s journey from idea to clinic
After starting as an idea on a whiteboard in Melbourne, the VividFlo glaucoma implant is now part of a landmark local clinical trial.
After starting as an idea on a whiteboard in Melbourne, the VividFlo glaucoma implant is now part of a landmark local clinical trial.
An innovative solution to protect the sight of people with moderate to severe glaucoma, designed by Melbourne company VividWhite, is now part of a large Australian clinical trial to determine its effectiveness and safety.
The VividFlo implant, designed by CERA Principal Investigator of Glaucoma Surgical Research Professor Michael Coote, represents a whole new way of managing glaucoma and a step towards improving how vision might be protected in the future.
“It has been incredibly rewarding to take this from an idea to a clinical trial, especially one that is so close to home,” says Professor Coote.
Managing options
For people whose glaucoma cannot be controlled through eye drops, surgery to reduce the pressure of fluid in their eye can become a necessary step to protect their sight.
There are several options to do this, from using a laser to improve the eye’s natural drainage pathways to using surgery to create additional paths for fluid to drain, but they are not effective for everyone.
Professor Coote’s work to find a new way started in 2010 when he pioneered a research project to better understand how fluid can leave the eye.
Seven years later, he founded VividWhite with experienced medtech executive Andrew Batty. VividWhite set about developing a device to meet a set of specific requirements and created the first prototypes for the VividFlo implant.
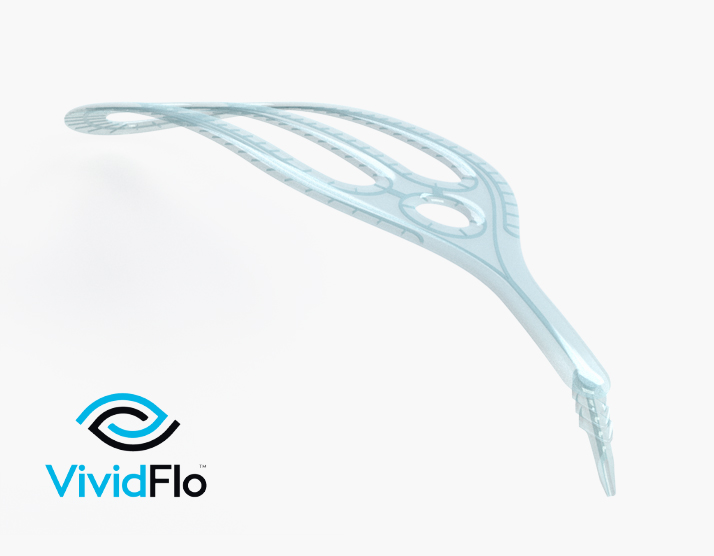
The implant is designed to create an additional channel in the eye through which fluid can drain to reduce pressure, and then disperse it gently through a series of over 150 exit channels spread out over the back of the eye.
This innovative design incorporates micro fluidics and nanofabrication to release fluid in a controlled and consistent fashion to protect the rest of the eye and potentially improve how glaucoma is managed long term.
Following the successful feasibility study in 2022, private investment and $1 million in funding from MTP Connect – the Australian Government’s Life Sciences Innovation Accelerator – the device is now part of a large, multi-centre study.
The trial is currently underway at both CERA’s Cerulea Clinical Trials and several other sites across the country.
Local benefit
CERA Managing Director Professor Keith Martin is one of the surgeons involved with the clinical trial, and said it’s been exciting to see a locally designed device come to trial.
“There are not many cases where a small team, without assistance from a major medical device or pharmaceutical company, have taken an idea like this all the way from the drawing board to clinical trial,” says Professor Martin.
“It’s quite a privilege to be part of this trial, based on such fantastic research and a design that has been entirely locally created.”
CERA researcher Dr Jennifer Fan Gaskin is the principal investigator at Cerulea coordinating the clinical trial and is also excited to be a part of it.
“As a surgeon, we’re always looking for better options to help patients manage their glaucoma and seeing this come to trial we hope we’ll soon have another.”
Professor Coote hopes that the device will improve the lives of the approximately 300,000 Australians affected by glaucoma.
“We’re aiming for the VividFlo implant to play a critical role in the suite of ways we can protect people’s sight from glaucoma.”

