This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Research
Research reveals genetic signature of macular degeneration
Better diagnosis and potential precision treatments for age-related macular degeneration (AMD) are a step closer, thanks to the discovery of new genetic signatures of the disease.
New research published in Nature Communications this week identifies 439 molecular signatures associated with AMD, with 43 of those being potential new gene variants.
The findings are the result of a long-running collaboration between CERA’s Principal Investigator Clinical Genetics Professor Alex Hewitt (who is also Professor of Ophthalmology at the Menzies Institute for Medical Research), Professor Alice Pébay from the University of Melbourne and Professor Joseph Powell from the Garvan Institute of Medical Research.
Other CERA researchers including Head of Macular Research Professor Robyn Guymer AM, Head of Mitochondrial Biology and Disease Dr Isabel Lopez-Sanchez, Dr Helena Liang, Lisa Kearns and Linda Clarke also contributed to the paper.
The research, which uses reprogrammed stem cells to create models of diseased eye cells and then analyse DNA, RNA and proteins, has the potential to lead to better diagnosis and treatment of the disease.
This could include matching patients to the treatment that will most effective for the specific genetic profile of their disease.
“We have been building a program of research where we’re interested in stem cell studies to model disease at a very large scale to do screening for future clinical trials,” says Professor Hewitt.
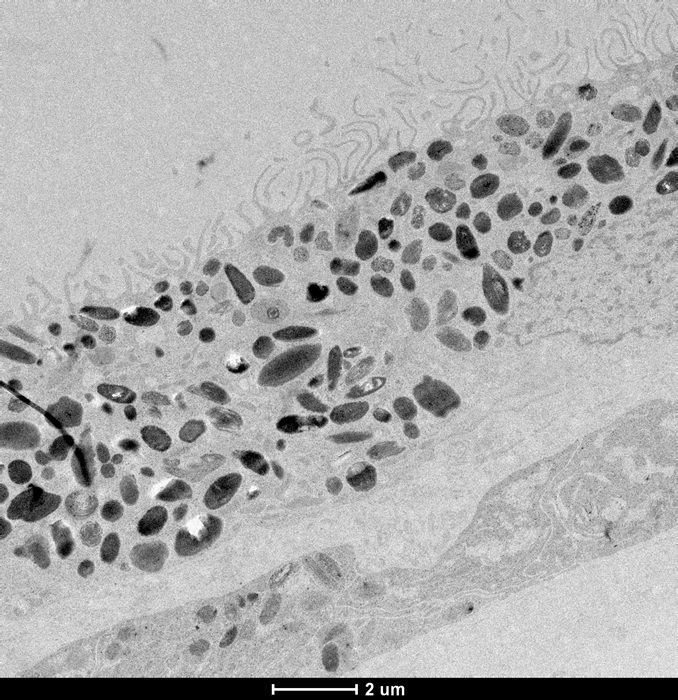
Cellular reprogramming
Age-related macular degeneration is the progressive deterioration of the macula – a region in the centre of the retina and towards the back of the eye – which can led to the loss of central vision.
Currently there are no approved treatments for the dry form of the disease.
For the study researchers took skin samples from 79 participants with and without the late stage of AMD, called geographic atrophy.
Their skin cells were reprogrammed to revert to stem cells called induced pluripotent stem cells, and then guided with molecular signals to become retinal pigment epithelium cells, which are the cells affected in AMD.
Retinal pigment epithelium cells line the back of the retina and are essential to the health and functioning of the retina. Their degeneration is associated with the death of photoreceptors, which are light-sensing cells in the retina that transmit visual signals to the brain and are responsible for the loss of vision in AMD.
Analysis of 127,600 cells revealed 439 molecular signatures associated with AMD, with 43 of those being potential new gene variants.
Key pathways identified were subsequently tested within the cells and revealed differences in the energy-making mitochondria between healthy and AMD cells, rendering mitochondrial proteins as potential targets to prevent or alter the course of AMD.
The molecular signatures can be used for screening of treatments using patient cells in a dish.
“Ultimately, we are interested in matching the genetic profile of a patient to the best drug for that patient,” says Professor Pébay.
“We need to test how they work in cells relevant to the disease.”
Professor Powell says the work tested the way that differences in people’s genes impact the cells involved in age-related macular degeneration.
“At the smallest scale we’ve narrowed down specific types of cells to pinpoint the genetic markers of this disease,” he says.
“This is the basis of precision medicine, where we can then look at what therapeutics might be most effective for a person’s genetic profile of disease.”
Read the study
Senabouth et al. Transcriptomic and proteomic retinal pigment epithelium signatures of age-related macular degeneration, Nature Communications (2022) https://doi.org/10.1038/s41467-022-31707-4
Funding
The research was funded by the Macular Disease Foundation Australia, Ophthalmic Research Institute of Australia, Retina Australia, DHB Foundation, The Goodridge Foundation, National Health and Medical Research Council, Australian Research Council, Medical Research Future Fund. CERA receives Operational Infrastructure Support funds from the Victorian Government.
CERA is grateful to the generous philanthropy of the Joan and Peter Clemenger Foundation and estate of the late Philip Neal which funded the essential infrastructure to support our IPSC stem cell research.

